Mutations
Background
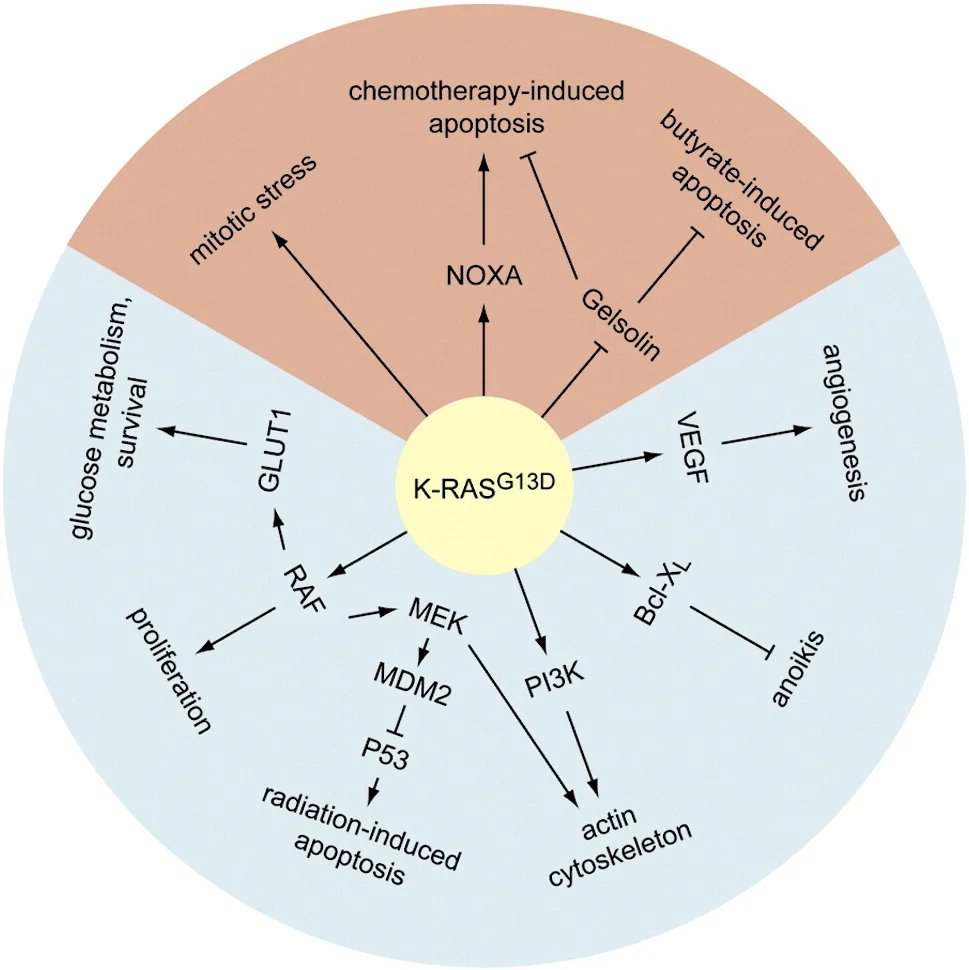
This year, it is estimated that 140,000 people will be diagnosed with colorectal cancer and about 50,000 people will die of the disease in the United States. Activating mutations in the RAS family of GTPases are common in human cancers. The RAS proteins (K-RAS4A, K-RAS4B, N-RAS and H-RAS) are members of a superfamily of small, monomeric GTPases. Upon activation by specific receptor tyrosine kinases, RAS proteins function as binary molecular switches that control intracellular signaling pathways such as actin cytoskeletal integrity, proliferation, differentiation, cell adhesion, apoptosis, and cell migration. Single amino acid mutations at codons 12, 13, or 61 were identified almost 30 years ago. These mutations essentially lock all RAS proteins into a chronically active (GTP-bound) state and therefore they are oncogenic. In colorectal cancers, mutations in K-RAS predominate while mutations in H-RAS and N-RAS are rare. While mutations at codons 12 and 13 account for the majority of activating mutations in K-RAS, weak-activating mutations at codon 146 (typically an alanine to threonine substitution) occur in approximately 4% of colorectal cancers and therefore outnumber strong activating mutations at codon 61 (1%).
Approach
Various colorectal cancer studies have indicated that K-RAS mutations at codons 12 or 13 are associated with poor prognosis, while mutations at codon 146 are associated with a more favorable clinical outcome in patients with colorectal cancer. Identification of a K-RAS mutant that would prove to be a potential target for therapeutic intervention in colorectal cancer would clearly be valuable.
- Using gene targeting we have generated mice expressing mutationally activated K-Ras A146T to examine the phenotypic consequence of this mutation in the intestinal epithelium.
- Using human colorectal cancer cell lines we will also assess how downstream signaling pathways are altered by recurrent mutations at codon 146 in K-RAS.